The linker of nucleoskeleton and cytoskeleton (LINC) complexes are molecular structures that span the nuclear envelope and connect the nuclear lamina to the cytoskeleton. The LINC complexes are composed of SUN domain proteins on the inner nuclear membrane and nesprin proteins on the outer nuclear membrane. Somatic cells express two SUN proteins, SUN1 and SUN2, that have both overlapping and specific functions. SUN proteins interacts with several nuclear envelope proteins, including emerin and lamins. Their interaction with lamins anchors the LINC complexes. Nesprins are more diverse. Not only because there are four nesprin genes expressed in somatic cells, but also because of these genes express many different isoforms. Nesprins interact with nuclear envelop protein emerin, and there are reports that different nesprins may interact with each other. But the major function of nesprins is to interact with cytoskeletal elements in the cytoplasm. Nesprin-1 and 2 interact with both actin and microtubule cytoskeleton. Nesprin-3 interacts with plectin, which is an adapter protein that can link to all three classes of cytoskeletal filaments. Nesprin-4 binds to the microtubule motor, kinesin.
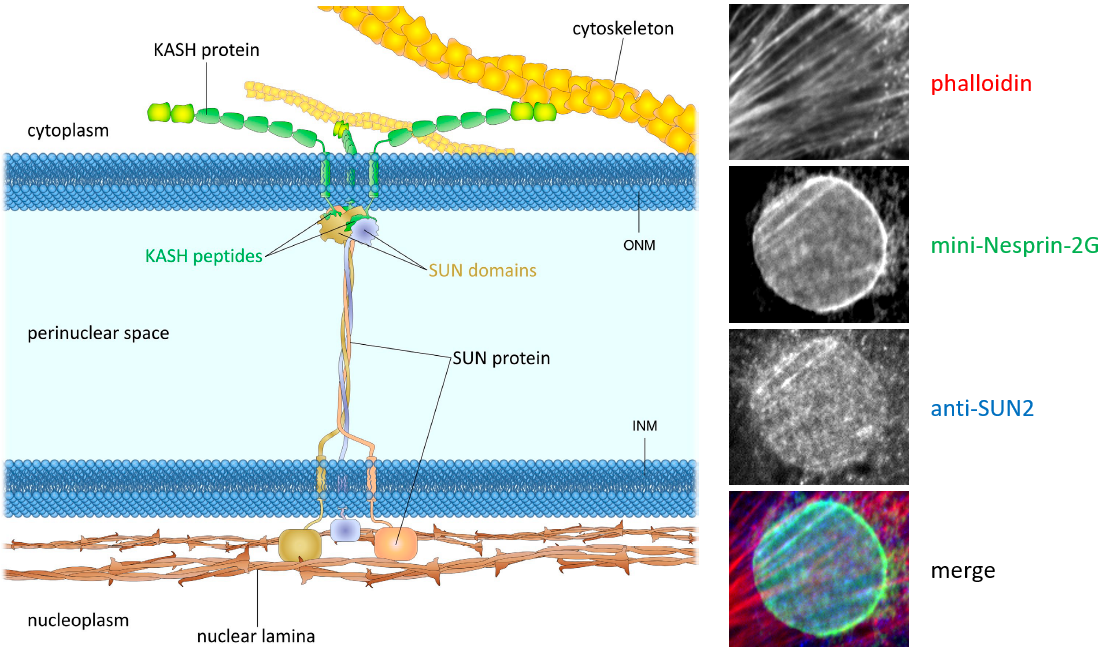 Left: from , JCB 2015.
Left: from , JCB 2015.
During nuclear movement in fibroblasts, nesprin-2G, the giant isoform of nesprin-2, capture the moving actin cables. Multiple nesprin-2 molecules thus align along actin cables and form a linear structure underneath the cable. This structure, termed transmembrane actin-associated nuclear (TAN) line, transfers force from the moving actin cables to move the nucleus. Interestingly, only SUN2, but not SUN1, is found in this structure, indicating that either interaction between actin and nesprin-2 affects nesprin-2’s binding to the SUN proteins, or SUN-nesprin binding regulates nesprin’s interaction with the cytoskeleton.
TAN lines contains other proteins like emerin, Samp1 and FHOD1. One other protein, lamin A/C, is not enriched in TAN lines but is required for the anchorage of TAN lines. In the absence of lamin A/C, TAN lines form but slip over the nucleus without moving it. Below is a movie of a cell expressing GFP-progerin, a mutant form of lamin A. In this case the lamina is weaken and cannot resist the force from actin cables (red, mCherry-LifeAct), GFP-progerin was torn apart from the lamina, became enriched on TAN line and moved along with it.
Reference
- . Samp1 is a component of TAN lines and is required for nuclear movement. J Cell Sci, 2012 Mar; 125(Pt 5):1099-105.
- . Emerin organizes actin flow for nuclear movement and centrosome orientation in migrating fibroblasts. Mol Biol Cell, 2013 Dec; 24(24):3869-80.
- . Accessorizing and anchoring the LINC complex for multifunctionality. J Cell Biol, 2015 Jan 5; 208(1):11-22.
- . Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. Proc Natl Acad Sci U S A, 2011 Jan; 108(1):131-6.
- . LINC complex proteins in development and disease. Curr Top Dev Biol, 2014; 109:287-321.
- . FHOD1 interaction with nesprin-2G mediates TAN line formation and nuclear movement. Nat Cell Biol, 2014 Jul; 16(7):708-15.
- . Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science, 2010 Aug; 329(5994):956-9.